After ADC, the Next Breakthrough — ABC Published in Nature Biotechnology
Over the past decade, antibody–drug conjugates (ADCs) have emerged as a powerful targeted cancer therapy, delivering cytotoxic payloads to tumor cells with high specificity. ADCs have demonstrated strong clinical efficacy and safety across multiple tumor types.
However, traditional ADC formats are limited by restricted drug-to-antibody ratios (DAR, typically 2–8), narrow payload scope, and limited control over drug release mechanisms, which have constrained their broader clinical potential.
To overcome these challenges, researchers at Massachusetts Institute of Technology recently published a breakthrough in Antibody-bottlebrush prodrug conjugates for targeted cancer therapy, unveiling a novel Antibody–Bottlebrush Prodrug Conjugate (ABC) platform.
By conjugating antibodies to a bottlebrush prodrug (BPD) architecture, ABC achieves an ultra-high DAR of up to 135 — 10 to 100 times higher than conventional ADCs — significantly expanding the payload landscape and unlocking new therapeutic possibilities.
Structurally, the ABC is composed of an antibody covalently linked to a bottlebrush prodrug (BPD). The BPD acts like a “molecular brush,” allowing for the flexible conjugation of a wide range of payloads—including clinically established ADC payloads such as MMAE and SN-38, traditionally incompatible molecules like DOX (doxorubicin) and PTX (paclitaxel), as well as next-generation modalities such as PROTACs (e.g., ARV771) and imaging probes (e.g., Cy5.5).

Fig 1. Construction and in vitro evaluation of ABCs.
Experimental data showed that ABC maintains HER2 binding affinity comparable to trastuzumab and exhibits markedly enhanced internalization and cytotoxic activity in HER2⁺ cells.
The researchers incorporated the BET protein degrader ARV771 into the ABC framework, creating the world’s first PROTAC-ABC conjugate. The results demonstrated that ARV771–HER2 exhibited robust in vivo antitumor activity at doses significantly lower than those required for free PROTAC, providing a promising new strategy to overcome the long-standing pharmacokinetic and delivery challenges associated with PROTAC therapeutics.

Fig 2. Efficacy and safety of ABCs incorporating the
PROTAC payload ARV771 in HER2+ BT-474 tumor-bearing mice.
Overall, the ABC platform, with its highly modular architecture, customizable design, and ultra-high drug loading capacity, effectively overcomes multiple limitations of traditional ADCs. It not only revitalizes the therapeutic potential of low-potency payloads but also offers a powerful delivery solution for next-generation modalities such as PROTACs. ABC is poised to become a key enabling technology for next-generation targeted cancer therapies, with strong potential for clinical translation.




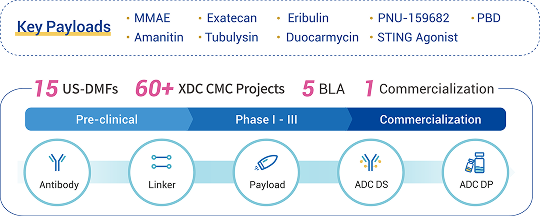
Reference:
1. https://www.nature.com/articles/s41587-025-02772-z