Tides CDMO Services
-
Amid the global shift toward more diverse and precise drug development, emerging modalities—such as peptides, oligonucleotides, Peptide-Oligonucleotide Conjugate (POC), and etc.—are demonstrating strong growth momentum and significant clinical potential. The market is expanding at a CAGR exceeding 10% and is projected to surpass USD 14.2 billion by 2034*, establishing itself as a strategic, high-growth therapeutic category shaping the future of the pharmaceutical industry.
ChemExpress is committed to supporting global pharmaceutical innovators with comprehensive, end-to-end Tides CDMO solutions. Leveraging a model built on efficiency, reliability, and scalability, we support our clients’ projects from R&D to commercialization.
* Polaris Market Research: Peptide and Oligonucleotide CDMO Market Share, Size, Trends, & Industry Analysis Report


Enabling Technology
Expertise in solid-phase peptide synthesis and liquid-phase synthesis, fmoc method, cyclic peptides, and peptide–drug conjugates (PDCs), supporting full-scale development from milligram (mg) to kilogram(kg)

Expert Scientific Team
10+ years of end-to-end expertise in peptides and oligonucleotides from R&D to commercialization, supported by a professional team of 50+ specialists
Global Compliance
ISO9001-aligned R&D and GMP-compliant production, meeting FDA/EMA/NMPA regulatory standards for worldwide market readiness
Our Services
ChemExpress provides end-to-end tides CDMO services from R&D to commercialization, with full-Time equivalent (FTE)/ fee-for-service (FFS) and CMC/CDMO services.
Peptide
Peptide High-throughput Synthesis
- 36/48 Multi-Channel Peptide Synthesizer
- Simultaneous Synthesis of 48 Peptides of Different Sequences
- Sequence Length: 3-60 AA
Customized Services
- Solid-phase Synthesis and Liquid-phase Synthesis
- Linear Peptides Synthesis
- Cyclic Peptides Synthesis
- PDC Drugs
- GLP-1 Derivatives
Quality Control
- Impurity Studies
- Chiral Impurity Analysis
- MS/MS-sequencing, N-sequencing, NMR, Amino Acid Composition, Corresponding Isomers, Assay, Solvent Residues, Heavy Metals, Microorganisms, Endotoxins, etc.
Oligonucleotide
RNA
- Small Interference RNA (siRNA)
- MicroRNA (miRNA)
- CRISPR sgRNA
- Small Active RNA (saRNA)
DNA
- Antisense Oligonucleotides (ASO) i.e. PS modified Gamer
- Aptamer
- Vaccine Adjuvant
Conjugated Oligonucleotide Sythesis
- GalNAc-oligonucleotide Conjugates
- Peptide-oligonucleotide Conjugates
- Antibody-oligo Conjugates
Our Sites
R&D Site & Manufacturing Site

Case Study
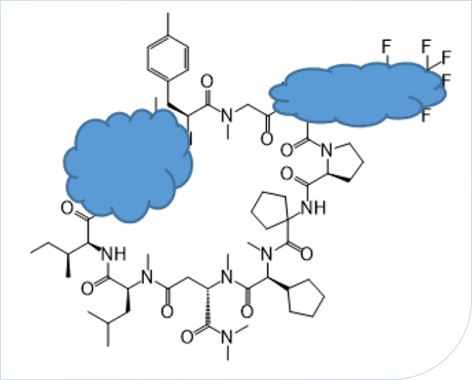
Cyclic Peptide Process Optimization
This project involved a cyclic peptide featuring a highly complex structure with multiple unusual amino acid residues. Due to significant steric hindrance and the challenging amino acid composition, the conventional condensation and cyclization reactions exhibited low efficiency and generated numerous impurities, creating obstacles for process scale-up and purification.
Leveraging its extensive peptide chemistry expertise, ChemExpress conducted in-depth literature research and designed a fragment-based synthesis route tailored for the unique amino acids. Systematic optimization was performed on key reaction parameters—including coupling reagents, solvent systems, reaction concentration, and time—at both the condensation and cyclization stages.
As a result, the optimized process achieved a significant improvement in cyclization conversion and product purity, while effectively minimizing byproduct formation. The refined process provided a robust foundation for subsequent scale-up and formulation development.
FAQs
Our peptide synthesis platform integrates solid-phase and liquid-phase synthesis, with high-throughput and automated production lines for rapid screening and synthesis. We can synthesize peptides ranging from 3 to 80 amino acids and offer synthesis scales from milligrams to kilograms. We also support the development of cyclic peptides (including polycyclic structures), fluorescent labeling, and other functionalized peptides.
Chiral impurities refer to non-target stereoisomers formed during peptide synthesis as a result of racemization or other causes. These impurities can impact the efficacy, safety, and stability of peptide products. Particularly in clinical applications, such impurities may lead to reduced drug activity or potential side effects.
The control of chiral impurities is achieved through the following strategies:
· Starting Material Control: Ensuring high purity of starting materials and setting strict quality standards to prevent introducing impurities from substandard materials.
· Process Control: Screening optimal reaction conditions (such as temperature, solvent, pH) to minimize racemization and reduce unwanted chiral impurities.
· Process Strategy: Implementing specific process strategies, such as using chiral reagents or enzyme-catalyzed reactions, to reduce racemization risks and ensure high chiral purity in the final product.
Structural characterization of peptides is essential to ensure product quality and functionality. We employ a range of analytical techniques, including nuclear magnetic resonance (¹H-NMR, ¹³C-NMR), mass spectrometry (MS), tandem MS sequencing (MS/MS), N-terminal sequencing, two-dimensional NMR (2D-NMR), infrared spectroscopy (IR), and ultraviolet spectroscopy (UV). These complementary approaches verify the structure, sequence, and purity of peptides, ensuring accuracy and reliability.